
Pharma’s next breakout opportunity isn’t yet another drug — it’s generative AI. Over the next six years, AI spending in pharmaceuticals is expected to increase by 600%, with 95% of companies already investing in some capacity.
This surge in adoption is driven by a simple but powerful proposition: faster, smarter, and safer operations. Gen AI is helping pharma companies accelerate the development of new treatments, enhance trial design, improve manufacturing quality, and personalize patient care — all while driving down operational costs.
In this post, we explore eight of the most promising generative AI use cases in pharma that are reshaping how pharmaceuticals are discovered, developed, and delivered.
7 Top Generative AI Use Cases in Pharmaceuticals
Industry leaders like Sanofi, J&J, Pfizer, and Moderna already have gen AI tools running at their research and manufacturing facilities. But most are only scratching the surface of what gen AI can do.
The McKinsey Global Institute (MGI) estimates that gen AI can add another $60 billion to $110 billion a year to the pharma and medical-product industries.
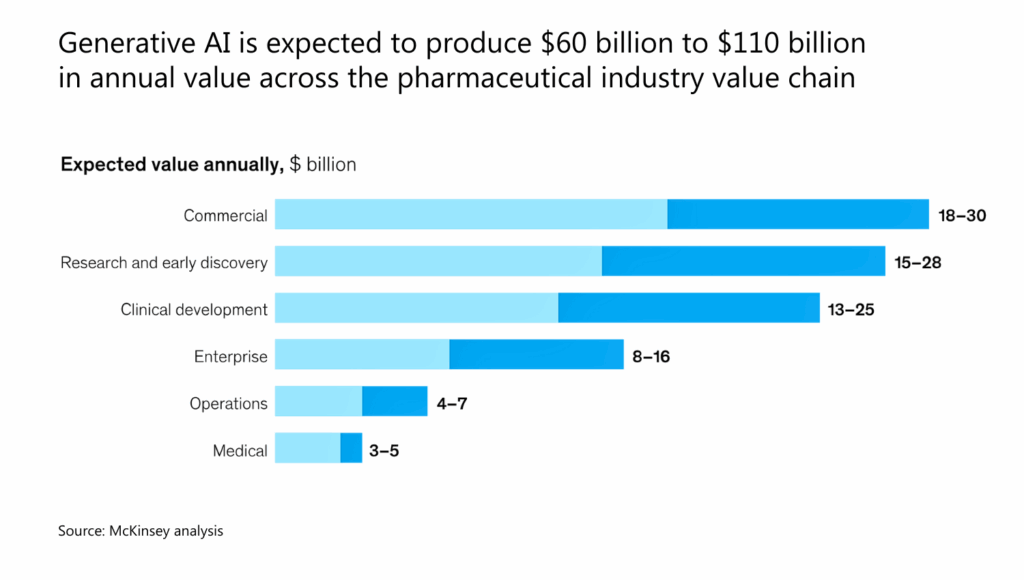
Source: McKinsey
That economic value comes not from a single silver-bullet application, but from dozens of high-impact use cases across the drug development lifecycle. Below are eight areas where generative AI is already making a measurable difference — from molecule to market.
Drug Discovery
The average cost to develop a new drug is a steep $314 million and $2.8 billion. Many of these costs get passed on to consumers, who then struggle to afford treatments. Research shows that higher out-of-pocket costs often lead to poorer medication adherence, which, in turn, drives worse health outcomes and more hospital visits. And that only increases healthcare costs.
Generative AI accelerates drug discovery by designing molecules with desired properties, cutting both time and cost compared to traditional methods. So that companies can combat “asset lifecycle compression” — the shrinking window to maximize a drug’s value before generics hit. According to McKinsey, that window has shortened by nearly 18 months over the past two decades, dropping from 11.7 to just 9.8 years. For consumers, this could mean faster access to innovative therapies.
Sanofi has become one of the leaders of AI-powered drug development through a landmark partnership with Formation Bio and OpenAI. By combining its biological expertise with Formation Bio’s clinical data and OpenAI’s large language models, the company has built a powerful platform for accelerating therapeutic design. In 2023 alone, Sanofi used AI to uncover 90 new targets and move seven into development, with 75% of its small-molecule portfolio now shaped by AI insights.
Eli Lilly and Takeda partnered with Anima Biotech on AI drug development. The biotech company has developed an mRNA Lightning.AI platform that uses neural networks to analyze images of cellular activity, distinguishing healthy from diseased cells and identifying disrupted pathways. These insights help uncover novel drug targets, all backed by lab validation. The company currently has 20 preclinical programs in neuroscience, oncology, and immunology.
Novartis chose Generate Biomedicines as its partner for developing novel protein therapeutics with the help of AI. Named as one of the private companies “upending the classic definition of disruption,” Generate developed advanced AI models for generating protein complexes of any variety, including antibodies that target defined epitopes and functional antibodies that agonize cell surface receptors. Their most successful AI-generated drug candidate, GB-0669 (a viral neutralizing antibody targeting SARS-CoV‑2) went from computer to clinic in just 17 months.
These early successes hint at a much larger shift ahead. As generative AI models become more powerful and better integrated with lab workflows, drug discovery could move from a slow, high-risk process to a faster, more precise, and commercially scalable engine for innovation.
Clinical Trials Optimization
Clinical trials — a key step in market entry of new drugs — take 6 to 7 years on average across Phases I to III. And the full cycle — from discovery to regulatory approval — takes up to 15 years on average (or more due to red tape). In the meantime, countless patients are left waiting for better treatment options.
Gen AI has the potential to streamline clinical trial design by identifying suitable patient populations, predicting outcomes, and optimizing trial protocols. Even more immediate wins lie in automating regulatory submissions, a task that traditionally takes weeks or even months.
Meanwhile, areas like patient recruitment, medical data analysis, and clinical operations are evolving steadily as gen AI builds on existing predictive tools, enhancing, not overhauling, the trial process.
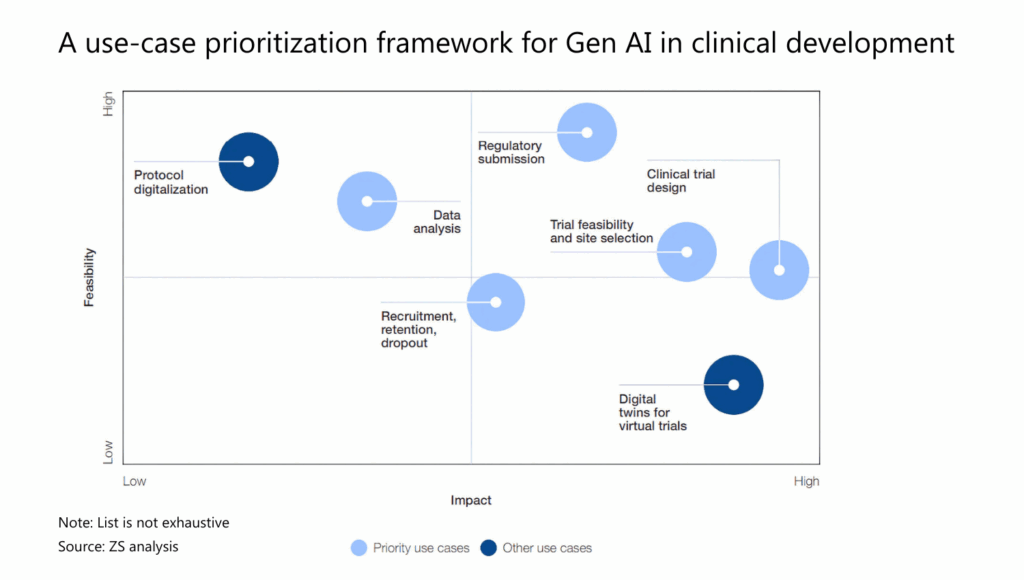
Source: WEF
AI has already “proven its chops” during COVID-19 vaccine development. Pfizer used AI to streamline its COVID-19 vaccine trials and accelerate distribution. The algorithms sifted through millions of data points from a 44,000-participant study, quickly identifying key signals to guide decision-making.
To further reduce time spent on data discovery, Pfizer equipped 1,500 scientists with generative AI, built with Amazon Bedrock and Anthropic’s Claude 2.1. They enable natural language and voice-based document search. Traditionally, developing a single drug can generate around 20,000 documents, requiring manual data hunts. With gen AI, Pfizer expects to save up to 16,000 scientist hours annually and cut infrastructure costs by 55%.
Moderna also relies on AI tools (including OpenAI models) to streamline clinical trial processes. Since early 2023, the company has scaled up to over 750 GPTs used in functions ranging from research to manufacturing, streamlining tasks like dose selection and regulatory documentation. One standout, Dose ID, uses advanced analytics to assess vaccine dosing, generate visual insights, and support safer, faster clinical decision-making in late-stage trials. This AI-driven approach enhances productivity while helping Moderna advance its mRNA medicine mission.
As AI accelerates both drug discovery and clinical trial execution, it’s also laying the groundwork for something even more transformative: personalized, precision medicine.
Personalized Medicine
Big data analytics in healthcare has already changed the degree of treatment personalization. Gen AI can take this a notch further through ongoing medication and treatment plan customization, plus more proactive patient support.
For instance, a conversational AI assistant that reviews a cancer patient’s genetic profile and adjusts their treatment plan weekly based on new lab results. Or a chatbot that monitors a chronic illness patient’s symptoms in real time, alerting doctors and suggesting medication tweaks before issues escalate.
Such scenarios are rapidly becoming the reality thanks to trailblazers like BenevolentAI. The startup collaborates with pharmaceutical companies on identifying drug candidates tailored to genetic markers with AI tools. BenevolentAI has already identified strong candidates for treating conditions like Parkinson’s and certain types of cancer.
Swiss Sophia Genetics, in turn, uses AI to integrate genomic, radiomic, and clinical data, enabling oncologists to craft more personalized cancer treatments. With over two million patient profiles analyzed, its platform delivers insights grounded in real-world data. Clinicians at CHU Lyon, one of France’s largest hospitals, report faster, more accurate diagnoses for hereditary cardiac conditions thanks to SOPHiA DDM™, making precision medicine more accessible and impactful at scale.
Gen AI is opening the door to a new generation of personalized digital therapeutics (DTx). AI-driven solutions are already capable of analyzing hundreds of patient-specific variables—genetic markers, behavior patterns, lifestyle data—to create individualized treatment algorithms that go far beyond what manual analysis can achieve. From adaptive VR/AR therapy programs to always-on conversational agents that guide patients through their care plans, gen AI brings deeper personalization, greater engagement, and real-time support to digital health experiences.
Synthetic Medical Data Generation
The healthcare industry is ultra-reliant on data, but it can be hard to come by due to privacy laws and security concerns. Regulations like HIPAA and GDPR limit medical data sharing, while fragmented systems and inconsistent data formats make integration even harder. The result is a slow healthcare digital transformation and limited accuracy of AI models.
Generative AI solutions can be trained to produce synthetic datasets that closely mirror real patient data, to then be used for training other models or conducting simulations. Researchers already agree that synthetic data can be a better alternative in sectors like healthcare. It enables faster research, model training, and hypothesis testing by providing readily available, privacy-safe data. In areas with limited real-world data, like rare diseases, it helps fill critical gaps, supporting broader studies and more targeted treatments.
RYVER.AI and SEGMED recently teamed up to build AI models for generating synthetic medical images (e.g., MRI, CT scans). Their combined approach promises more accurate AI models for medical imaging, supporting better disease detection and more inclusive care. This collaboration also benefits pharma companies by enhancing real-world evidence (RWE) studies for faster clinical trials and advanced precision medicine initiatives.
A Medidata and Cornell University team also recently suggested a new method for producing synthetic clinical trial data. Their tool, Simulants, uses key variables like endpoints and patient characteristics from historical clinical trials to produce new anonymized data. Unlike traditional anonymization, it breaks records into fragments and recombines them to create privacy-safe datasets that retain the integrity of the original data, offering researchers a secure, realistic alternative for analysis and AI model training.
With further advances in generative models, synthetic medical data quality will become even better, enabling even more possibilities for drug development, diagnostics, and personalized care, with zero compromises on patient privacy.
Compliance Management
Pharma companies have a close-knit relationship with regulators: they interact daily on document exchanges, regulatory submissions and quality audits. These interactions consume a lot of operational costs and the team’s time.
Gen AI tools can streamline some aspects of compliance management:
- Automate document generation and reviews
- Summarize complex regulatory guidelines into plain language
- Optimize clinical trial compliance
- Flag discrepancies and potential non-compliance in real time
- Track changes in global regulatory frameworks
- Generate risk mitigation strategies for specific jurisdictions
- Facilitate cross-departmental knowledge sharing
Merck KGaA, for instance, launched an internal ChatGPT assistant that’s integrated with enterprise resource planning (ERP) systems. It automates tasks like drafting procurement orders and quality reports using real-time SAP data, checks content against GMP standards to flag compliance issues, and summarizes technical documents to streamline collaboration, cutting meeting prep time by 25%.
Thanks to retrieval-augmented generation (RAG), you can cost-effectively adapt general-purpose LLMs to specialized tasks like pharma compliance management or medical summarization. RAG works by pulling relevant information from connected business systems or databases in real time, grounding the model’s responses in domain-specific knowledge without retraining. Our AI software engineering team can develop RAG-based assistants within 3 months for a variety of use cases.
Quality Control
Pharmaceutical manufacturing processes are inherently complex, and many things can go awry at different stages — milling, granulation, blending, drying, compression, and coating. This increases the average Cost of Poor Quality (COPQ) in the pharma sector to about 25-40% of annual turnover.
AI can aid with the costly deviation management by providing contextual guidance and corrective actions in real-time. Such systems also enable predictive insights for proactive quality assurance. For example, computer vision-based systems can flag at-risk batches before production errors occur and perform root cause analysis to help with process correction.
Roche, for instance, built an AI-powered system for predicting trends in titer, yield, and critical quality attributes (CQAs). It fine-tunes process settings, detects deviations early, and improves batch sequencing. According to the Roche data analytics team, the new system drove up to 10% higher yields, 50% fewer CQA write-offs, and deeper insights that drive future product innovation.
The pharma company also uses a RAG-based assistant to analyze historically similar deviations and address production issues more effectively. By using prompt engineering and predictive insights, Roche has moved from reactive troubleshooting to proactive quality control, cutting manual review time by up to 50% and speeding up issue resolution across manufacturing operations.
Workplace Knowledge Management
Lastly, generative AI tools can add extra efficiency to other operating workflows — operations, marketing, sales, and more. From drafting investor reports to pulling insights from large datasets and automating routine documentation, Gen AI helps pharma teams work faster and smarter.
Pfizer built a generative AI platform for its marketing division. Charlie helps medical review teams prioritize content by flagging assets that need closer scrutiny, like new claims or reused language in new contexts, while skipping over repetitive or low-risk materials. It’s evolving into a full marketing workbench, integrating media analytics, competitor insights, and platform tools like Adobe Workfront and Slack to streamline workflows and boost cross-team collaboration.
Bayer, in turn, rolled out myGenAssist — a private, in-house generative AI platform tailored to the needs of its 40,000+ employees. It can generate reports, presentations, and visuals, cutting content production times in half for marketing teams.
Users can also parse proprietary research, regulatory files, and clinical trial data using simple language queries, which has slashed information search time by 30% in R&D. myGenAssist also automates repetitive tasks like data entry, invoice handling, and compliance tracking, with supply chain teams using it to produce real-time inventory updates and predictive maintenance plans.
Conclusion
Generative AI is the flagship focus for pharma companies, especially as the first ROI starts to roll in. But to turn early wins into lasting impact, you need more than standalone AI pilots — you need a focused adoption strategy tied to both scientific and business outcomes. That means building an end-to-end tech stack for AI solutions development, investing in the infrastructure to support scaled systems, and prioritizing data security.
Successful adopters start small, testing validated AI use cases through MVPs. Before scaling these, they tackle legacy data infrastructure and implement governance frameworks like FinOps to track spending, forecast ROI, and avoid hidden costs.
The next phase won’t just be about what Gen AI can do, but how well companies manage the complex orchestration behind it. Edvantis would be delighted to further advise you in this area. As a full-cycle engineering partner, we offer tailored support at every stage of model development, as well as in ancillary areas like cloud management, data analytics, and cybersecurity. Contact us for a personalized consultation.






