Around the world, millions of people are waiting to receive the care they need. Yet, public healthcare systems are overburdened and private healthcare is only accessible to a fraction of those patients.
On the other hand, patients who do receive detailed diagnoses then struggle to implement the recommended lifestyle changes, follow the prescribed medication routine, and show up for regular follow-up visits, especially when it comes to chronic disease management. Healthcare providers, in turn, lack the ability to monitor the patient’s compliance levels and don’t have the data they need to create personalized regimens.
Digital therapeutics have emerged as a new HealthTech domain, focused on addressing the above problems.
What is Digital Therapeutics (DTx)?
Digital therapeutics (DTx) is a new category of evidence-based, patient-facing software and hardware applications that help deliver remote care for a wide range of diseases and disorders.
With the help of DTx, healthcare practitioners can monitor patients at a distance and receive extra data on their health to develop more personalized treatment plans. A number of DTx applications aim to change the patient’s behavior, helping them adopt better habits, and effectively manage chronic symptoms.
Examples of digital therapeutic solutions include:
- Mobile applications
- Medical IoT devices
- Health wearables
- AR/VR apps
Overall, DTx products can complement or augment the value of the traditional healthcare delivery system. In some cases, DTx can also replace certain in-person therapeutic interventions. For example, RelieVRx designed an FDA-authorized in-home VR treatment program for patients with chronic lower back pain, which can replace in-person physical therapy sessions.
Although DTx is a part of the digital health domain, such products are different from diagnostics software or telehealth platforms. They are used to deliver treatment, not just to advise medical professionals.
Unlike consumer-grade wellness apps and wearable devices, digital therapeutics have to pass extensive clinical validations and obtain respective regulatory approvals. They must be based on scientific evidence and demonstrate efficacy in supervised clinical trials.
According to the Digital Therapeutics Alliance, all products claiming to be digital therapeutics must follow a set of foundational standards.

Source: Digital Therapeutics Alliance
Benefits of Digital Therapeutics
DTx has already been proven effective for a variety of healthcare applications in clinical trials. For example, medication adherence among patients using DTx apps is 80%, which is higher than that of pharmacotherapy (50%) alone.
A clinical study of an FDA-approved mobile app for treating substance abuse has also found that 40% of patients who used a DTx app stayed sober for 3 months versus 17.6% of those who used standard therapy alone.
Generally, the key digital therapeutics benefits include:
- Better access to clinically proven and effective therapies
- Higher degree of treatment personalization
- Continuous monitoring of patients’ vitals and progress
- Improved adherence to recommended treatment plans
- Significant scalability potential to extend clinical care
- Multi-language support, tailored to the patient’s preferences
- Private access to treatments that are often stigmatized
- Data-backed insights into patient behavior and treatment effectiveness
- Higher cost-effectiveness compared to traditional treatments
- Great potential for delivering preventive care
Given that healthcare systems in many countries are overloaded and hospitals face critical staff shortages, DTx could help address the gaps in coverage by providing patients with complementary care at scale.
Digital Therapeutics vs. Software as a Medical Device (SaMD)
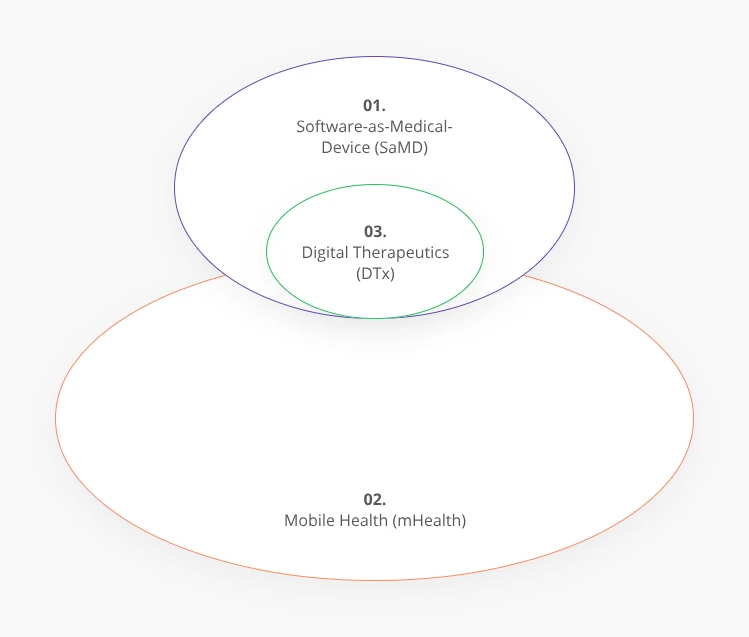
In the wider context of the HealthTech ecosystem, DTx is a subset of Software as a Medical Device (SaMD) and overlaps with Mobile Health (mHealth) solutions.
Source: Springer Link
The International Medical Device Regulators Forum defines Software as a Medical Device (SaMD) as “software intended to be used for one or more medical purpose(s) that perform(s) these purposes without being part of a hardware medical device.”
SaMD can be a standalone solution or used in combination with other medical hardware devices (e.g., an X-ray system), medical software (e.g., EHR/EMR systems), or general-purpose software. For example, a radiology image recognition software, used for cancer detection is considered SaMD.
Since DTx is based on software applications, they qualify as Software as a Medical Device. However, unlike SaMDs, digital therapeutic apps are patient-directed and they can be prescribed to patients as a treatment option.
Some digital treatment programs can be delivered via a mobile app, meaning such DTx solutions will fall into the broad term of mHealth. The Global Observatory for eHealth defines mHealth as “medical and public health practice supported by mobile devices, such as mobile phones, patient monitoring devices, personal digital assistants (PDAs), and other wireless devices.”
mHealth solutions can include some DTx applications but are generally more focused on preventive medicine (e.g., an assistant app reminding a diabetic patient to monitor their sugar intake). One of DTx’s key interfaces is mobile applications, but it can also be delivered via non-mobile technologies. Also, mHealth solutions, unlike DTx, are typically not subject to regulations and don’t have to be validated in clinical trials.
Effectively, DTx occupies a nexus position between consumer-grade wellness apps and wearable devices on one side and safety- and quality-controlled Software as a Medical Device on the other.
Main differences between SaMD, DTx, and mHealth products
| Software-as-a-medical device (SaMD) | Digital therapeutics (DTx) | Mobile health (mHealth) | |
|---|---|---|---|
| Patient- or therapist-directed | Both | Primarily patient-directed | Both, but offer a consumer-grade experience |
| Medical purpose | Universal – support multiple use cases | Diagnostic and therapeutic purposes | General health and wellness purposes |
| Regulatory aspects | Applicable medical device standards and regulations; data protection regulations | Applicable medical device standards and regulations; data protection regulations, plus further regulations, covering safety, efficacy, and security of the pruduct. | For health data processing: general data protection regulation (GPDR) in Europe, HIPAA in the US |
Source: Springer Link
Digital Therapeutics Market Overview
Digital therapeutics may sound like a new term, but the underlying concepts date back to 1995 and the research of Dr. Joseph Kvedar from Boston, USA. Kvedar was working on developing a conceptual “one-to-many model of care” and looking into ways to expand the physicians’ ability to accommodate a growing number of patients with the help of technology.
In 2015, Dr. Cameron Sepah, an Assistant Clinical Professor in UCSF Medical School’s Department of Psychiatry, used the term “digital therapeutics” for the first time in a paper about delivering web-based care to diabetes patients. He defined DTx as “evidence-based behavioral treatments delivered online that can increase healthcare accessibility and effectiveness.”
The first commercial DTx products have started to emerge around the same time. An attractive value proposition and high market demand propelled strong investment support. Between 2011 and 2018, the investments in digital therapeutics companies in the US grew by 40% year-on-year.
Between 2020 and 2021, the funding for DTx projects increased by an astounding 133% and reached $3.4 billion total.
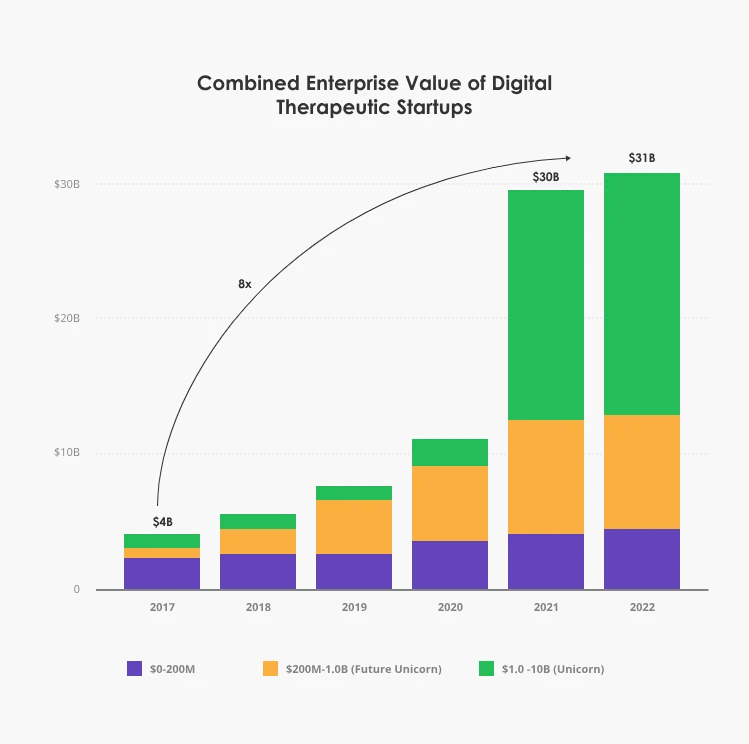
Source: Dealroom.
In Europe, German Kaia Health and Sidekick Health from Iceland are on track to become potential future unicorns. A Swiss neurorehabilitation startup MindMaze and a diabetes treatment company Diabeloop are also in the spotlight after raising $105 million and €70 million last year, respectively.
In the US, Hinge Health, specializing in digital solutions for managing joint and muscle pain, reached a $1 billion valuation after the last funding round. Click Therapeutics, in turn, got an FDA-approval for its mobile application for smoking cessation, which showed high efficacy in clinical trials.
Global governments also positively view DTx as another element in their plans to digitize healthcare systems. Respectively regulatory frameworks are already in place in the United States, Asia (Japan, South Korea, China, and Singapore), and several European countries (France, the UK, and Germany).
Germany added DTx to its universal healthcare reimbursement program in 2019. The National Health Service (NHS) in the UK funds the development of new DTx products and works on a new proposal for including Prescription Digital Therapeutics (PDTs) into the reimbursement scheme.
With high interest from patients and clinical professionals alike, and increasing support from the governments, DTx is a promising niche for HealthTech product development. Although the entry barrier is high, the competition is still relatively low with many untapped value pools.
4 Digital Therapeutics Use Cases Worth Exploring
New digital therapeutic products are being developed to tackle a wide range of diseases, ranging from type II diabetes and chronic pain to anxiety, depression, and substance abuse.
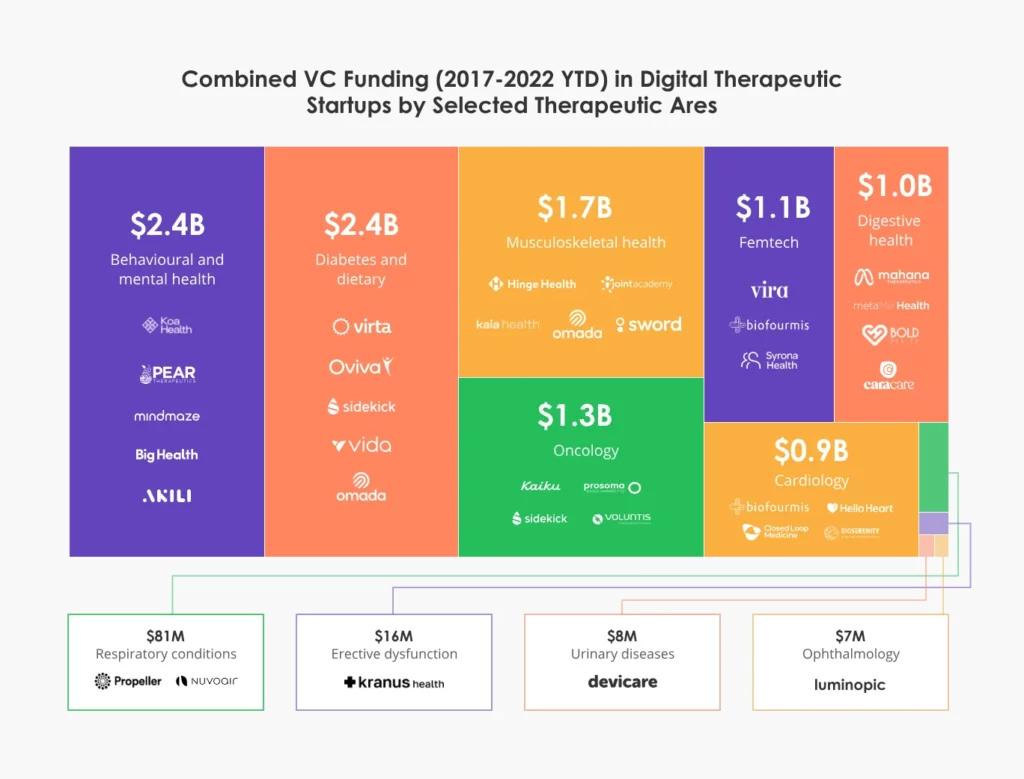
Source: Dealroom.
Chronic Disease Management
Chronic health conditions require ongoing care. However, with the growing number of patients, healthcare systems often cannot cope with the demand.
Over 133 million Americans suffer from at least one chronic disease. Nearly half of Germans, aged 50–59 years, have two or more chronic health conditions. Many more people globally remain undiagnosed because they can’t receive timely access to care.
Chronic diseases need to be managed not only by medication but also with regular monitoring and lifestyle changes — and that’s where DTx can help.
Neptun by Orbit Health, for example, is an AI-driven product to help better monitor Parkinson’s patients’ symptom progression and help them better manage treatment-induced side effects. By combining sensor technology and analytical algorithms to continuously collect motor symptom and treatment response insight, so that clinical professionals could provide more personalized patient care.
Fitterfly, in turn, created a unique diabetes digital therapeutics product, which uses sensing and mobile technology to develop personalized diabetes care programs. Fitterfly has helped over 20,000 individuals better manage diabetes and reduce obesity. The average patient achieved a 1.96% in HbA1c levels; improved weight, sleep quality, and stress levels. The startup now plans to focus on developing an AI-powered coaching assistant to answer patient queries, provide routine information, track progress monitoring, and send reminders.
Digital Therapeutics For Mental Health
Plenty of mental health apps are available on the market, but few have proved to be effective in clinical settings. Unlike your regular meditation app, DTx for mental health is evaluated by regulating bodies and clinically tested, before being approved for usage.
Respectively, such products can be used to effectively treat widespread, yet often stigmatized, mental health issues such as anxiety, depression, obsessive-compulsive disorder, panic attacks, and post-traumatic stress disorders among other ailments.
Digital therapeutics examples in this category include clinical products like My IBD Care’s new digital therapy courses for treating general anxiety disorder among people with Chron’s disease. During a study held between January 2021 and March 2022, the team measured how the participants measured on the General Anxiety Disorder (GAD) PROM (Patient Reported Outcome Measure test. DTx courses started and completed were strongly correlated with high cumulative GAD PROM scores. Meaning that higher engagement with DTx courses reduced anxiety among users. An earlier study also showed improvements in disease control and generalized anxiety levels among patients after two weeks of using the app.
EndeavorRx created a gamified DTx product for improving attention function in children with attention-deficit/hyperactivity disorder (ADHD). The product uses sensory stimuli and motor challenges to stimulate specific cognitive functions. A clinical study shows that EndeavorRx product helped substantially improve objective attention in children with ADHD between 8 and 12 years.
Freespira developed a digital solution for handling panic attacks. By combining sensing technology, physiological feedback, and coaching Freespira helps patients alleviate PTSD symptoms and reduce the frequency of anxiety attacks. Over 90% of product users reported a clinically significant reduction in panic symptoms over one year.
Prescription digital therapeutics (PDTs)
Prescription digital therapeutics (PDTs) are software-based therapies, requiring authorized administration from a healthcare professional. Patients cannot use them independently and need clinical oversight.
On the regulatory side, such products must meet the following requirements:
- Have an evidence-based mechanism of action
- Pass randomized controlled trials
- Be authorized or approved as safe and effective
- Have reimbursement pathways via specific product codes
In the US, nine PDTs are currently authorized to treat conditions such as substance use disorders, attention-deficit disorder, and chronic insomnia such as Nightware, Mahana IBS, RelieVRx, and Luminopia One.
Lumniopia recently received an FDA-approval for its VR-based headset therapy for amblyopia (Lazy Eye). Patients can choose to watch over 700 hours of popular TV shows and movies, adjusted to promote eye-sight correction. The program is backed by extensive neuroscience research and has been evaluated in three clinical trials.
Globally, prescription digital therapeutics examples include WELT-I — a six-week cognitive behavioral therapy (CBT) for insomnia treatment, which was recently approved by the South Korean government after a successful clinical trial.
DTX for Oncology
For many patients, cancer is an ongoing battle. Both treatable and terminal cases require careful adherence to supporting therapy to ensure a better quality of life. Different DTx products have been already found effective in improving patient outcomes.
A two-year clinical study found that lung cancer patients who were using DTx had almost twice as high overall survival rates — 22.5 months in the digital intervention group vs. 14.9 months in the control group. Another study at the Memorial Sloan Kettering documented a median five-month extension of life among solid-tumor cancer patients, along with substantial improvements in quality of life.
In oncology, digital therapeutics can also help enlist more patients in clinical research as such solutions reduce the entry barrier and make studies more accessible to various participants. DTx also allows more objective data collection as it minimizes the “white coat” effect, which negatively impacts data quality.
For example, Kaiku Health (now Elekta Kaiku) is already helping oncologists collect real-world patient data and provide timely responses. The platform’s algorithm screen and prioritize symptoms, alert care teams, and provide guidance to patients. The company also entered a new partnership with Novartis last year to develop a new digital therapy module for patients receiving anti-cancer medicines. The key objective of the project is to develop machine-learning-based algorithms for symptom prediction and personalizing symptom management for Novartis cancer medicine, plus obtain new insights on patient outcomes.
Final Thoughts
Digital therapeutics is a rapidly evolving segment of the HealthTech market. It has a substantial depth with large patient populations suffering from the lack of accessible chronic disease treatment options, over-burdened state health systems, and cost-inhibitive private care options.
The challenge of entering this market, however, is that you need to balance the “clinical” side of product development with stellar digital experiences. DTx products must meet regulatory standards and showcase clinical efficacy, but they must also appeal to users in terms of ease of use, convenience, and connectivity. Poor app UX/UI, poorly scalable platform architecture, and system bugs will affect your products’ adoption rates as much as the lack of solid scientific evidence.
Edvantis helps global companies engineer competitive eHealth solutions. With over 10 years of experience in the industry, we know how to balance regulatory requirements with attractive product design. Learn more about our healthcare software development services or contact our team directly for a personalized presentation.

